Title:
Evaluation of the effect of different formulation on suppository characteristics.
Evaluation of the effect of different formulation on suppository characteristics.
Objectives:
To
study the effect of different composition of base on the physical
characteristic of suppositories.
Introduction:
Suppository is a solid formulation
of different sizes and shapes suitable for rectal drug administration. Good
suppository will melt after the rectal administration and release the drug
either topically or systematically.
The drug needs to be dispersed in
suitable suppository bases. Good bases are not toxic, no irritation, will not
interact with other drugs and also easy to be mould into a suppository.
Different composition on base will affect the rate and limit of release of the
drug from the suppository
In this experiment, the effects of
the different base composition to the suppository physical characteristics and
also to the drug release characteristics are evaluated.
Apparatus:
Analytical
balance, weighing boats, spatula, 50ml and
100ml beaker, hotplate, 5ml measuring cylinder, suppository mould, water bath
37oC, dialysis bag, glass rod, 5ml pipette, plastic cuvette,
spectrophotometer UV/Vis
Materials:
Polyethylene
glycol (PEG) 1000, Polyethylene glycol (PEG) 6000, paracetamol, distilled water
Methods:
1. Paracetamol saturated stock solution
is prepared by adding 10g of Paracetamol in 5ml distilled water.
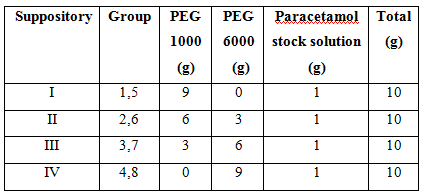
2. The 10g suppository is prepared
using the formulation below:
3. The suppository is shaped using the suppository
mould. The shape, texture and color of the suppository is observed and
discussed.
4. One of the suppositories is placed
in the water bath 10ml at 37oC and the time for the suppository to
melt is recorded.
5. Another suppository is placed inside
the dialysis bag and placed in the 50ml beaker. The beaker then placed inside
the water bath 37oC.
Dialysis bag
6. The sample is pipette in 5 minutes
interval and the release of the Paracetamol from the suppository is determined
using the spectrometer UV/Vis. The distilled water must be stirred first before
the sample is taken.
Results:
Figure
1: Physical appearance of suppositories
Discussion:
1. Compare and discuss the physical appearance
of the suppository formed.
Basically the shapes of all
suppositories are torpedo because we used similar type of mould. Based on
smoothness and hardness of the suppositories comply with the standard
suppository which has smooth surface and perfect hardness. The suppositories
are not too hard which may cause bad disintegration process and not too soft
which may cause problem when insert the drug into the rectum area. The
different in oily surface of suppository of each group may due to the amount of
liquid paraffin used during lubricating the mould. The colour of the
suppository that used high amount of PEG 1000 is more even white compare to
suppository that used high a amount of PEG 6000. This is due to the
characteristics of the PEG itself, PEG 1000 is more whitish and smooth compare
to PEG 6000. In conclusion, to the ingredient used is the main cause that
contribute to the physical characteristics and the rate of the drug release via
rectal route.
2. Plot
a graph of the time needed to melt the suppository vs. the amount of PEG 6000
in the formulation. Compare and explain the results.
PEG
6000 is a suppository base and theoretically, increasing the mass of the PEG
6000 will make the suppository more solid. Thus, the time taken for the
suppository to melt should actually increases with the amount of PEG 6000 in
the suppository.
Based on the results we obtained, the longest time is used to melt the
suppository that has the highest amount of PEG 6000, which is 9g. This does
comply with the theory. However, the results deviate for the suppositories that
contain 0 and 3g of PEG 6000 g. For these 2 suppositories, the average time
needed decreases with the increasing amount of PEG 6000. This shows that the
results we obtained is inappropriate.
The deviation of the result from the story is majorly effected by the errors
occur while conducting the experiment. Defect of suppository made reduction in
mass and will reduce the time for suppository to dissolve. Error made during
measuring and transferring the ingredient while making suppository may also alter
the results. There is also a possibility that suppository does not solid enough
when we taken out from the refrigerator. The unsolidified suppository made it
easier to be dissolved in water bath. Another causes may due to some of the
group might stir the beaker containing suppository which make it faster to
dissolve.
3. Plot
a graph of UV absorption against time. Analyse it.
This
experiment was used to determine the absorption rate of the suppository in
human body. In this experiment, the dialysis bag represents human biological
membrane while distilled water represents human blood plasma. This experiment
is carried out in water bath at 37ºC, which represents human body temperature. Due
to the concentration gradient, the high concentration of water molecules of
distilled water will diffuse into the dialysis bag whereas the high
concentration of particles in paracetamol suppository diffused out of dialysis
bag into distilled water. The paracetamol suppository melted at 37 ºC.
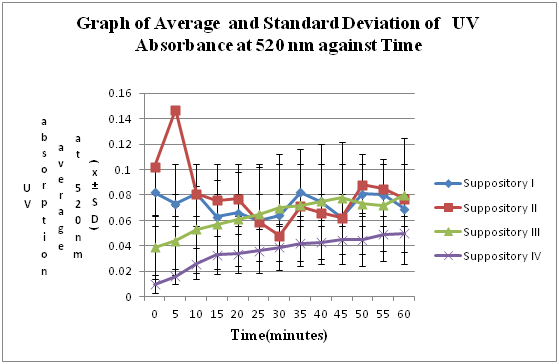
The graph
shows that the UV absorption is increasing with time. It indicated that the
amount of paracetamol released into distilled water is increasing with time.
The longer the time, the higher amount of paracetamol released to the distilled
water. Theoretically, a sigmoid graph will be obtained in this experiment due
to the constant drug release rate until reaches the equilibrium. However, the
graph we obtained shows the inconstant release of paracetamol. This happened
due to some errors in this experiment. One of the errors is the uneven stirring
of distilled water before it is taken for the measuring of results. Besides
that, the water bath may not maintain at 37 ºC. Next, the distribution of
paracetamol in the suppository is not homogenous. This is due to the air bubble
trapped inside the suppository during the preparation of suppositories. This
leads to the alteration of the drug release rate. Lastly, the reading was not
taken at exactly 5 minutes interval. All of these lead to the inaccuracy of the
results.
4. Plot
graph of UV absorption against time for the suppository formulation with
different compositions. Discuss and compare the results.
There are 4
parameters of an in vitro suppository drug release, which are
temperature, contact area, release medium and membranes. Throughout the
experiment, 37oC is used for the experiments of drug release from
the suppositories. Measurements of drug release at 37oC may become
an over estimation due to the differences in melting range of the
suppositories.
There
is no apparatus to mimic the contact area between the suppository and the
rectal mucosa. This is important in determining the rate of drug release of the
suppository. As long as this is not developed, the results are not tailor-made
for the real condition, inaccurate results may be produced.
Distilled water is
considered as the release medium in this experiment. However, we should know
that there is no ideal solution yet due to the problem of choosing a suitable
volume and composition of the release medium that suits the condition in the
rectal area.
The
“membrane” we used in this experiment is the dialysis bag. This may come with
an enormous drawback as the release measured in the outer compartment is not
equal to the actual release that is taking place in the inner compartment. The
membrane may form a resistance to passing drug molecules. The actual release
may be underestimated.
Based on the graph,
there is a wide range of variation which do not correlates with the theoretical
principle. Theoretically, suppositories that are prepared with the different
combination of PEG 1000 and 6000 show different release rate of drug against
the time that contribute to solubility and dissolution of drugs in the aqueous
medium. Hence, the graph obtained for the four different formulations of
suppositories should be increasing initially and then become constant gradually
as all the drug is released into the water and diffuses out from the
hydrophilic matrix with time. And, the suppository with the highest amount of
PEG 6000 will show the slowest release rate due to the stronger hydrogen bonds
formed with Paracetamol substances which hinders the release of Paracetamol.
Decreasing the high molecular weight PEG (6000) concentration and increasing
low molecular weight PEG (1000) concentration in the base resulted enhancing
the in-vitro release of the drug and vice versa. Water solubility of the drug
suppository increases as the molecular weights of PEG decrease due to the water
absorbing properties of PEG. Thus, the highest rate of release is expected for
suppository I due to the lowest proportion or amount of PEG 6000 in the
formulation while formulation suppository IV with higher contents of PEG
6000 will give the slowest releasing rate of drug due to the strong hydrogen
bond among molecules PEG 6000 with molecules Paracetamol. The UV absorption will increase
with time until it reaches a plateau stage where the entire drug has been
released.
However, from the
graph obtained, suppository II shows the higher rate of drug release than
suppository I which is deviates from the theory. In fact, the suppository II
has higher concentration of PEG 6000 and lower concentration of PEG 1000 that
should have lesser drug release rate than I. Suppository II shows the higher
rate of drug release than suppository III as it has higher composition of PEG
1000 and lower amount of PEG 6000 that slow the release rate of drug. This
obeys to the theory.
Deviations or inaccuracy occurred in
the experiment may be due to impurities, parallax error, and
equipment used give inaccurate readings, uneven temperature of the water bath and others. Existence of
impurities results from improper cleaning of the cuvette for assay. Cuvettes
that are not properly dried before we insert a new sample for assay may affect
the readings. Dialysis bag that is not tied well before inserting into water
bath leads to the fluctuation readings. Besides, inaccuracy of readings may
also caused by the obtained sample form unstirred solution in beaker before
analysis.
Therefore, several precautions
should be taken during the experiment likewise ensuring the cuvette is dried
completely before inserting into the absorption spectrophotometer. We should
stir the solution containing sample in the beaker before taking the sample for
analysis. Lastly, ensure that our beaker containing the sample is always
maintained at even temperature in water bath. These precautions may cut down
the deviations formed from errors in conducting experiments.
5. What
is the function of every substance used in this suppository preparation? How
can the different contents of PEG 1000 and PEG 6000 affect the physical
characteristics of the formulation of a suppository and the rate of release of
drug from it?
Ingredients that used in this
experiment are Paracetamol, PEG 1000 and PEG 6000. The Paracetamol acts as the
active ingredient in this suppository. It is the main substance in this
formulation which contributing to the therapeutic effects. While PEG is
Polyethylene Glycol which acts as suppository bases in this formulation.
Polyethylene glycols are polymers of ethylene oxide and water prepared to
various chain lengths, molecular weights, and physical states. They are
available in a number of molecular weight ranges, the most commonly used being
polyethylene glycol 300, 400, 600, 1,000, 1,500, 1,540, 3,350, 4,000, 6,000,
and 8,000. PEG are water soluble or water miscible type of bases that commonly
used as suppository bases in pharmaceutical industry. It is because the special
characteristic of PEG that has low melting point that suitable to be in body
temperature. Various active ingredients can be dissolved in PEGs and have a good
bioavailability. They act as carrier bases, solubilisers and absorption
improvers for the drugs. Suppository that used higher amount of PEG 6000 take a
longer time to release the active ingredient due to the melting point is around
56˚C to 63˚C while the melting point of PEG 1000 is in 37˚C to 40˚C range which
as body temperature range. However, for a better shelf life the combination of
PEG 1000 and PEG 6000 is important to produce slightly higher melting point of
suppository and not easily melt on the shelf.
PEG 6000 has higer molecular weight compare to
PEG 1000.Higher proportions of high molecular weight polymers produce
preparations which release the drug slowly and are also brittle. Less brittle
products which release the drug more readily can be prepared by mixing high
polymers with medium and low polymers. The PEG 1000 give very soft masses while
PEG 6000 will give more solid products. The use of different contents of PEG
1000 and PEG 6000 results in different effects on the physical characteristics of
the suppository produced and this will subsequently affect the rate of drug
released from the suppository. More hydrogen bonds are formed between the PEG
6000 molecules and drug molecules when the more PEG 6000 is used. This will
result in the increase of the hardness of the suppository and also the
difficulty of the drug released from the suppository. The production of
whitish, very hard, less sticky and very rough suppository will be obtained. On
the other hand, PEG 1000 produces whitish, very soft, most sticky, and very
smooth suppository. Thus, suitable and appropriate combination ratio of PEG
1000 and PEG 6000 is important in the production of an optimum drug delivery
with optimum bioavailability of drugs available to the body and also to avoid
too hard or too soft suppository.
Conclusion:
The suppository composition will affect the physical property and the
drug release rate from the suppository. The higher the amount of PEG 6000, the
suppository will be harder.
Reference:
- TorsenHennig, Polyethylene glycols (PEGs) and the pharmaceutical industry,
Fine,speciality and Performance Chemicals, June 2002











Spectrophotometer cuvettes Awesome article, it was exceptionally helpful! I simply began in this and I'm becoming more acquainted with it better! Cheers, keep doing awesome!
ReplyDelete